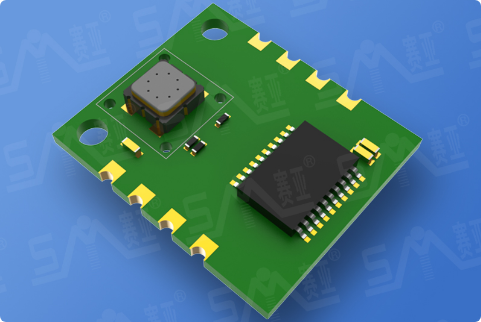
In lithium iron phosphate (LiFePO₄) cells undergoing thermal runaway, the decomposition of the solid electrolyte interphase (SEI) membrane, coupled with exothermic reactions between anode materials, cathode materials, and the electrolyte, generates hydrogen (H₂) and carbon monoxide (CO) gases at elevated concentrations. By employing high-sensitivity H₂ and CO gas sensors, these critical precursors can be detected in advance, enabling prompt mitigation measures to prevent or delay thermal runaway propagation.

During the thermal runaway process of lithium-ion cells, significant heat release occurs, causing the temperature to escalate from normal operating levels to extreme values up to 170°C. By deploying infrared temperature sensors with a measurement range up to 1000°C, precise real-time monitoring of cell temperature dynamics can be achieved. This enables prompt intervention through automated systems when predefined temperature thresholds are reached, effectively mitigating thermal runaway progression and enhancing safety protocols.
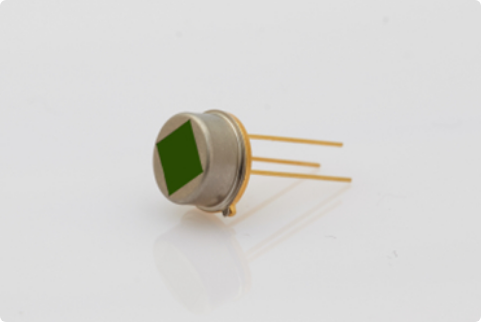
After the safety valve of a lithium iron phosphate (LiFePO₄) cell opens, continuous smoke emission typically occurs without visible flames. To prevent unintended thermal runaway triggered by accidental events, multi-band infrared fusion detection technology is employed to monitor potential flame ignition risks. This advanced sensing approach ensures real-time tracking of thermal and combustion precursors, enabling immediate response protocols to suppress hazards and enhance operational safety.

Aerosols achieve fire suppression and cooling through two primary mechanisms. On one hand, they utilize the endothermic decomposition of metal oxides and carbonates to absorb heat. On the other hand, they exert gas-phase and solid-phase chemical inhibition effects by engaging in multiple reactive interactions with key combustion intermediates - active radicals H, OH, and O. This dual-action process consumes and neutralizes substantial quantities of these high-energy reactive species, thereby effectively suppressing the combustion chain reaction.

Perfluorohexanone can evaporate rapidly within seconds, absorbing substantial heat energy to drastically reduce the temperature around the fire source and effectively contain flame spread. As it vaporizes, it forms an inert gas layer that blocks oxygen contact, further suppressing the combustion process to achieve rapid and complete fire suppression. This extinguishing agent also exhibits exceptional environmental performance. It leaves no residual deposits after application, preventing secondary pollution to both the environment and equipment, fully meeting modern firefighting requirements for green and eco-friendly solutions.